
EdPlace's Year 9 home learning science lesson: Separating Mixtures
Looking for short lessons to keep your child engaged and learning? Our experienced team of teachers have created English, maths and science lessons for the home, so your child can learn no matter where they are. And, as all activities are self-marked, you really can encourage your child to be an independent learner.
Get them started on the lesson below and then jump into our teacher-created activities to practice what they've learnt. We've recommended five to ensure they feel secure in their knowledge - 5-a-day helps keeps the learning loss at bay (or so we think!).
Are they keen to start practising straight away? Head to the bottom of the page to find the activities.
Now...onto the lesson!
Sieving through the information!
Seawater, a cup of coffee, pen ink – these are all mixtures. That means they are made up of more than one thing, and they can be separated into their components using different methods – some of these methods you’ve probably used in the kitchen without even thinking about it! We've tried to condense all the key information below for you on these different methods, so filter out the background noise and immerse yourself in the pure excitement of separating mixtures!
We're confident if you follow this step by step approach your child will be able to:
1) Understand the various methods used to separate mixtures
2) Apply this understanding and if they’ve really cracked it
3) Explain this back to you
Step 1: Technical Terminology
We are going to have to use a few scientific keywords when talking about separating mixtures, some of which you've probably heard before but some which might be new. It's important we understand the scientific meaning of them before we start using them, so here goes...
Mixture: contains different substances that are not chemically joined to each other
Filtration: the process of passing a mixture through a device where soluble substances pass through the filter as a 'filtrate' but insoluble substances will stay in the filter as a 'residue'.
Evaporation: the process in which a liquid changes state and turns into a gas.
Distillation: separation method used to separate a solvent (the substance that dissolves the other substance eg water) from a solution/mixture.
Fractional distillation: the method used when a mixture of several substances, such as crude oil, is distilled and each evaporated component is collected as they condense at different temperatures.
Boiling point: the temperature at which a substance changes from a liquid to a gas.
Condense: when a gas is cooled and changes into a liquid
Chromatography: used to separate dissolved solids dissolved in a liquid.
Step 2: What is a mixture?
Before we jump into the various ways of separating mixtures, it's first important to check we understand what a mixture is. We're all used to hearing the word mixture when making a cake – you add all the ingredients, mix it up and you have a cake mixture, which you then cook and devour (in a surprisingly short period of time if you’re anything like us!). A cup of coffee is made up of coffee granules, water, milk and sugar. The cup of coffee is a mixture, made up of these four components. But, how about other substances that we might not be so aware of? Seawater is a mixture made up of water and salt, and pen ink is a mixture, made up of various pigments suspended in a solvent.
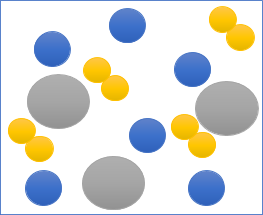
This picture shows a particle diagram of a mixture. Each of the circles represents a different substance – as you can see there are three different substances in this mixture. But they're not chemically joined together, they're separate which means they can be easily separated if you use the right method.
Step 3: Methods for Separating Mixtures
Some mixtures are not quite so easy to separate back out into their components, our cake mixture, for example. It would be quite tricky to get the eggs back out of that! But we do have methods we can use to separate lots of other mixtures, from the simple such as filtration and evaporation, to the more complex such as distillation and chromatography.
Filtration – You’ve probably used this method countless times in the kitchen before, without even realising it. Draining peas from their cooking water, using a tea strainer to collect your tea leaves when pouring your morning cuppa – these use filtration to separate the mixture into its components. The soluble liquid part runs through the filtering device (sieve, colander, filter paper) and the insoluble solid part is left behind. If we had some sandy water, we could use filtration to separate the sand particles from the water.
Evaporation – Again, you’ve probably used this method when cooking – have you ever made a sauce that’s just a bit too watery and so kept it on the heat to get it to thicken up? That’s evaporation, you apply heat to remove the water (it turns to vapour). It doesn’t have to be a quick process, the sun’s heat can be used to evaporate the liquid too. We could use this method to separate the salt from seawater, just leave it out in the sun long enough and you’d be left with dry salt crystals! Why not try it yourself with some really salty water at home? Pour it onto a baking tray and leave it out in the sun for a few hours, and hopefully, you’ll see the salt crystals appear if it's been a hot sunny day!
Distillation – Some mixtures are a bit more tricky to separate, but distillation works by separating the different components according to their boiling point (the point they turn from liquid to a gas). You need to heat the mixture, collect the gases that are produced at the different boiling point temperatures, before cooling them down quickly which causes them to condense back into a liquid. There’s a type of distillation called fractional distillation, which is used to separate a liquid from a mixture of two or more liquids.
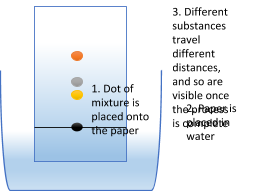
Chromatography – This process is used to separate dissolved solids from one another. You can use it when you have more than one solid dissolved in a liquid, it's useful for separating coloured substances. If you place a spot of the mixture on to a piece of chromatography paper and place it into a small amount of water, the different coloured substances will travel different distances up the paper, leaving multiple spots showing how many different substances there are in the mixture. The reason substances move different distances along the chromatography paper is all about their solubility in the solvent. The more soluble the particles are in the solvent, the further they travel up the paper. A pure substance will only produce one spot because it only contains one thing.
Why not have a go yourself using the basic techniques we’ve just covered? Cut a 15cm strip of kitchen roll, colour in the bottom 2cm of each end with the colours of the rainbow using washable pens. Get 2 cups of water and place just the bottom 1cm of each end of the kitchen roll in the water, and watch a chromatography rainbow appear!
Step 4: Show what you know!
Ok, now its time to see whether all this information is sinking in. Try these questions to check!
Distillation Dilemma...
Ribena is a drink made from blackcurrants. The blackcurrants are picked and taken to factories, where they are squashed and the resulting juice is separated from the berry skins. To make the blackcurrant juice into a concentrated form like Ribena, some of the water needs to be removed. Unfortunately, during this process, some of the particles that give the juice a blackcurrant smell also evaporate. Distillation is used to collect these ‘smell particles’ instead, as they have a boiling point of 50oC, whereas water has a higher boiling point of 100oC. The blackcurrant concentrate and the smell particles are mixed together when the Ribena is put into bottles, ready for you to buy.
-
What separation process do you think was used to separate the blackcurrant juice from the blackcurrant skins
-
Why can’t the water just be removed by evaporation?
-
Would simple distillation collect the smell particles if their boiling point was 100 oC? Explain your answer.
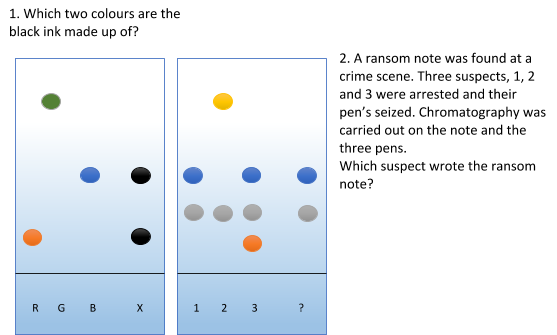
Chromatography Conundrum
Separation Station
What methods would you choose to separate the following mixtures?
a) The solute from a solution eg sugar from a sugar-water solution
b) Solvent from a solution eg water from a saltwater solution
c) Liquid A from a mixture containing three liquids A, B and C with different boiling points
d) Iron fillings from saltwater
e) Salt from a mixture containing salt, sand and water
f) Pure water from a mixture containing salt, sand and water
Step 5 - Apply the knowledge and practise
Now, you’ve covered this together why not put this to the test and assign your child the following 5 activities in this order.
All activities are created by teachers and automatically marked. Plus, with an EdPlace subscription, we can automatically progress your child at a level that's right for them. Sending you progress reports along the way so you can track and measure progress, together - brilliant!
Activity 3 - Substances: Separation Techniques
Activity 5 - Fractional Distillation
Answers
Distillation Dilemma:
-
Filtration.
-
Because the smell particles would also evaporate and be lost
-
No, because they would evaporate and condense with the water particles.
Chromatography Conundrum:
-
Red and Blue
-
Suspect 1
Separation Station:
a) Evaporation
b) Distillation
c) Fractional distillation
d) Filtration
e) Filtration then Evaporation
f) Distillation
g) Chromatography
Keep going! Looking for more activities, different subjects or year groups?
Click the button below to view the EdPlace English, maths, science and 11+ activity library

.jpg)






